
The Ultimate Guide to Implementing Quality Management
Table of Contents:
1) The Main Components of Quality Management
2) How to Effectively Implement a Quality Management System (QMS)
3) Four Best Practices for Handling Non-Conformances
4) Maintaining ISO 9001 Compliance
1. The Main Components of Quality Management
If you want to make sure you are providing consistent products or services throughout your organization, you must implement an ironclad quality management system, or QMS. Quality management ensures that what your company delivers, and the delivery processes it uses are cohesive and that every business phase of the organization focuses on the same goal.
When broken down, quality management can be segmented into four main components to be effective: quality planning, quality control, quality assurance, and quality improvement. Read on as we explain these four critical steps.
Quality Control Planning
The first step of the quality management process is planning. You need to take the time to identify your goals and what you want your baseline to be. You should determine what your quality standards are, the requirements necessary to meet these standards, and what procedures will be used to check that these criteria are being met.
Quality Control
Once you have a plan in place, quality control comes into play. This is the process of physically inspecting and testing what you laid out in the planning stage to make sure it is obtainable. You need to confirm that all the standards you have put into place are met, and you need to identify any mishaps or errors that need to be corrected. The sooner you can catch these errors, the better. As such, you should be paying attention to all aspects of the product, including both the materials used and the process of putting them together.
Quality Assurance
While quality control involves inspecting the actual products or services in the field, quality assurance is reviewing the delivery process of services or the quality management manufacturing of goods. By inspecting your goods or services at the source, you can catch mistakes before they reach the customer. You can also fine tune your processes to prevent errors in the future.
Quality Improvement
Finally, after completing the quality control procedures, you need to thoroughly review your findings and come up with a way to improve your methods going forward. Quality control management is fruitless if you are not willing to make changes when they are necessary. The desire for continual improvement is the goal for every successful company. So, gather all your data, re-evaluate both the processes and the product—always keeping compliance in mind—and then begin the quality control management process again. With each cycle, you will end up with a better product, happier customers, and more profit in your pocket.
2. How to Effectively Implement a Quality Management System (QMS)
A comprehensive Quality Management System (QMS) takes a critical look at your internal policies, processes, and procedures to help you increase efficiency throughout production, development, and service. Managing those processes and procedures can be time-consuming and overwhelming without a well-established system in place. Here are four steps you should take to effectively implement a quality management system at your company.
Define your quality objectives
Quality objectives describe your company’s goals for the value level of your products or processes. If you plan on implementing a QMS, you need predetermined targets to compare your actual finished goods to once they are produced. These objectives hold both your company and employees accountable to meeting a measurable value standard. There is a wide range of quality objectives, and they can range from department-specific, to ones that pertain to specific products or processes. Here are some examples of objectives to consider for your company and products:
- Defects
- Efficiency
- Performance
- Reliability
- Accuracy
- Customer service
Determine your training needs
To achieve your quality goals, your employees need to be trained on the policies and procedures that make up your quality management system. One of the most overlooked items of any quality plan is the adherence to policies and procedures by the people it’s written to protect. Moreover, one of the most targeted areas of a regulatory audit is the training records of everyone in the company that has a need to know and understand what they do and how they do it.
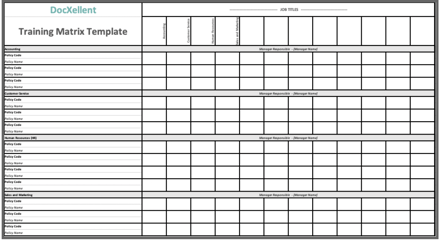
Developing a training matrix is one of the easiest and most organized things you can do for to assist in your compliance efforts. Establishing employee groups by department, job titles, having detailed job descriptions, identifying supervisors and subordinates are the employee piece. Then marrying that information with SOPs, policies and training curriculum per job title is the tedious but essential part. This can easily be done using a spreadsheet.
Click Here to Download DocXellent’s Auditor Approved Training Matrix Template
Monitor and implement activities to improve your performance
Regularly validating your QMS is a critical part of your quality management processes. The act of validating involves reviewing the delivery process of your services or the quality of your finished products to make sure they are meeting your pre-defined quality standards. By inspecting your goods or services at the source, you can catch mistakes before they reach the customer. You can also fine tune your processes to prevent errors in the future. When reviewing your processes in order to validate them, you will want to follow these steps:
- Confirm that everything is operating as it was agreed in your originally defined quality objectives
- Measure how effective your pre-determined processes are and confirm that all compliance needs are being met
- Take note of any lessons learned
- Identify areas where there is an opportunity for a smoother process
Employ corrective and preventive measures
Corrective Action Preventive Action (CAPA) refers to the process of inspecting and resolving the cause of identified non-conformances within your production processes. If your quality process is lacking in any way, CAPAs allow you to pinpoint an issue and solve it, preventing it from happening in the future.
In a successful CAPA process, there are certain data points that need to be documented and steps to be taken within each stage:
| Stage | Data Points |
| Identification |
|
| Investigation |
|
| Corrective Action |
|
| Preventative Action |
|
| Verification |
|
Under a quality management system, each employee works towards measurable objectives. Human errors are diminished, and workers have a straightforward idea of what their quality objectives are daily, ensuring that your organization isn’t wasting time and resources. And of course, less time and resources wasted leads to more money in the business purse.
3. Four Best Practices for Handling Non-Conformances
No matter how hard you work to maintain your quality standards, you are bound to encounter non-conformances at some point. A non-conformance in quality management is defined as any type of deviation from a specification, a standard, or a production expectation that indicates something went wrong along an assembly process. Here are four tips to help you better prepare for these instances:
1. Establish a system for supplier managementAlthough they may be a third-party organization, suppliers are a direct extension of your company. They provide you with the necessary parts, pieces, materials, and/or software to create your product. So, how well you manage your supplier processes plays a large role in the quality of your production line. If the supplies they offer are sub-par, delivery is consistently late, or you regularly receive the wrong materials, you’ll be facing non-conformances and customer complaints that are completely avoidable.
The first step in a well-managed supplier process is evaluating the vendors you choose to make sure they’re qualified. This evaluation begins before you start receiving materials and may include a survey of various questions to help you judge initial suitability.
Check out this supplier evaluation checklist template by Process.st
Once you’ve chosen a supplier and are receiving goods from them, it’s crucial to properly monitor and oversee them. Utilize documentation and software that allows you to track both orders and non-conformances per supplier. This way, if there are deviations from your standards that stem from supplier mistakes, you can handle them more efficiently and keep them from occurring again. A specification management system can make this process simple by allowing you to set up automatic notifications that let your suppliers know when specifications become effective or are updated. These spec systems create a transparency throughout your supply chain to ensure all stakeholders are working from the most up-to-date version of a spec.
Moreover, it is important to keep an open stream of communication with your suppliers. This means keeping them regularly informed and up to date on your strategy, procedures, and specification changes. This helps them know where they fit in your process and how they can help make your production line as efficient as possible. Prioritize making them your business partner; let them know which strategies are working and which aren’t. A stronger, deeper relationship with clear and frequent communication allows this interaction to become more organic and benefit both parties. This will mitigate non-conformance instances that stem from a lack of clear communication and understanding.
2. Document and analyze customer feedback
While all serious customer complaints are recorded and handled, customer feedback can also be a powerful tool in minimizing non-conformances. Taking notice of what customers are saying about your product helps you notice small issues that could turn into major non-conformances down the line and opens an opportunity for process improvement.
To take advantage of feedback, spend the time to fully analyze the statements your company receives. Studying what your customers say can help you spot trends and determine the root cause of performance issues, which is vital in preventing non-conformances. Understanding the root differentiates a singular lapse in quality from a process flaw that needs to be mitigated. Based on what your customers are saying, your company can determine which issues are worth looking into so that they don’t morph into serious issues. No system is perfect, problems along your production line are bound to occur. But by using customer feedback to your advantage, you can successfully prevent non-conformances before they happen.
3. Perform inspections during every part of your production process
Oftentimes preventing non-conformances starts with simple inspections throughout the production process. This means that before a product can even go through your manufacturing process, you need to outline inspection criteria for each stage of its journey; inbound, in-process, packaging, and shipping. If you don’t segment your inspection standards into each of these sections, you could be ready to ship a product and discover there is a quality management process issue that occurred three steps earlier. Non-conformances of these types can wreak havoc on not only your outbound schedule but also your bottom-line.
An important step in performing quality management product inspections is to define the critical dimensions of your inspection criteria. For example, you might have specifications that outline the dimensions your product is supposed to be. A quick check of a product during in-process will determine if your manufacturing is aligned with the proper dimensions and is thus meeting your quality standards. There are usually two or three critical dimensions like these that your inspector can check very quickly to determine if the part is good.
It is also important to understand that there might be different steps of the quality management process that require different levels of inspection, depending on the product you’re evaluating. If you have inspection standards in place, they need to be rigorous and complete. If your evaluations aren’t thorough, it will be easy to overlook possible quality issues that lead to more serious non-conformances down the line.
4. Conduct internal audits
Internal audits are a great way to prevent non-conformances by ensuring your company’s processes are meeting your quality standards. The overarching goal of an internal audit is to ensure that your company’s policies and quality control procedures are followed and to alert you of any holes in your standard compliance so that they can be resolved before they cause more serious problems.
Internal Audits need to be scheduled at regular intervals to check whether your manufacturing processes conform to the quality requirements your company has outlined. Any previous quality findings and past audit conclusions become valuable data moving forward in your quality process. Observations raised during internal audits could be classified as preventive actions as they can suggest improvements within the system to prevent non-conformances from occurring in the future. These audits will also help ensure your company is complying with regulatory standards, such as ISO 9001, further bolstering your overall quality process.
The biggest key to conducting an internal audit is to take it seriously and ensure the quality process is demanding. When looking for a third-party audit service provider, or when putting together your own internal auditing program, make sure that the audit will look through every aspect of your quality process. This will ensure that there are no holes in your findings, and you can feel relaxed knowing all your business quality control procedures are solid and you are preventing production mistakes wherever possible.
Effectively preventing and managing non-conformances is an essential part of an organization’s quality improvement plan. Less non-conformance instances results in fewer defective products and processes, resulting in more satisfied customers.
4. Maintaining ISO 9001 ComplianceA major goal of your quality outcomes is adherence to ISO 9001, and for some industries, it’s a legal or contractual requirement. ISO 9001 standards require organizations to define and follow a quality management process that is effective as well as expecting them to identify areas for improvement and take action toward those adjustments. As a result, it is typically understood that an organization claiming ISO 9001 certification is an organization with products and services that meet quality standards.
ISO 9001 standards are based on the plan-do-check-act method and provide a detailed approach to documenting and reviewing the responsibilities and procedures required to achieve effective quality management. Specific sections of the standard contain information on the following topics:
- Responsibilities of document management control
- Management of resources, including human resources and an organization’s work environment
- Product realization, including the steps from design to delivery
- Measurement, analysis, and improvement of quality management processes through activities like internal audits and corrective and preventive action
Use a QMS Software to Simplify ISO 9001 Compliance
Attempting to meet these ISO 9001 standards using manual methods can be a drain on resources. Businesses that are using paper, spreadsheets, or other manual methods of managing quality processes typically come to a point when investing in a Quality Management Software becomes necessary. Quality Management Software is a platform for storing, controlling, and automating your documents and processes to improve efficiency, quality, and compliance.
The table below outlines exactly how a QMS software can simplify your business’s compliance management by meeting each quality condition your company requires:
| ISO 9001 Requirements (informal interpretation) | Quality Management Software (QMS) |
| The quality policy is a formal statement from management, closely linked to the business and marketing plan and to customer needs. | Quality documents may be drafted, approved and controlled within your QMS. They may additionally be secured with print controls and watermarking. |
| The quality policy is understood and followed at all levels and by all employees. Each employee works towards measurable objectives. | Document Training enforces the reading and comprehension of documents in conjunction with quizzes within the quality system. |
| The business makes decisions about the quality system based on recorded data. | The QMS electronic Review cycle offers virtual collaboration. |
| The quality system is regularly audited and evaluated for conformance and effectiveness. | QMS’s managed and scheduled periodic review cycles allow for the evaluation of documentation. |
| Records show how and where raw materials and products were processed to allow products and problems to be traced to the source. | Binder and BOM templates offer the ability to link documents together to produce a ‘where used’ look up. |
| The business determines customer requirements. | Requirements may be drafted, approved, and controlled within your QMS. They may additionally be secured with print controls and watermarking. |
| The business has created systems for communicating with customers about product information, inquiries, contracts, orders, feedback, and complaints. | Configurable Distribution allows notifications and content to be emailed to users of the QMS system as well as external contacts. These emails are configured based on lifecycle events such as ‘upon Current and/or Historic’. |
| When developing new products, the business plans the stages of development, with appropriate testing at each stage. It tests and documents whether the product meets design requirements, regulatory requirements, and user needs. | A QMS’s comprehensive and configurable workflow allows for the incorporation of Action Items, Linking and Routing to allow for a full cycle of task initiations and completions. |
| The business regularly reviews performance through internal audits and meetings. The business determines whether the quality system is working and what improvements can be made. It has a documented procedure for internal audits. | Internal Audit forms may be generated within the QMS and electronically submitted for completion. This form data is then able to be extracted for reporting purposes. Additionally, Action Items and Periodic Reviews can tie into audit tracking. |
| The business deals with past problems and potential problems. It keeps records of these activities and the resulting decisions and monitors their effectiveness. | Incident tracking forms may be generated within your QMS and electronically submitted for completion or users may work from the built in Incident template. The system offers multiple lifecycle options that may be utilized to incorporate multiple departmental approvals and the resolution and closing of an Incident. Additionally, Action Items can tie into reporting. |
| The business has documented procedures for dealing with actual and potential non-conformances (problems involving suppliers, customers, or internal problems). | NCR forms may be generated within the QMS and electronically submitted for completion or users may work from the built in CAPA template which includes a Non-Conformance section. Your QMS will offer a multi-tiered CAPA lifecycle that may be utilized to incorporate multiple departmental approvals. Additionally, Action Items can tie into NCR reporting. |
|
The business:
|
CAPA forms may be generated within the system and electronically submitted for completion or users may work from the built in CAPA template. A quality management system offers multi-tiered CAPA lifecycles that may be utilized to incorporate continuous improvement processes including Root Cause identification, Analysis, Implementation, Acceptance and Verification. Additionally, Action Items can tie into reporting. |
Other Benefits of Quality Management Software
Reduces human error
When handling the quality management process, many companies still rely on manual data entry and other functions that are prone to human error. A QMS quality control software eliminates the need for paper data entry and turns a two-step process into one simple step. Workers can collect data on a convenient electronic device in real time and diminish the risk of common transfer errors.
A QMS can reduce both the frequency and the severity of human errors using the following capabilities:
- Documents can be drafted, approved, and controlled within the software where there is a complete version history of who did what and when to every document
- Create electronic forms where you can structure your data with controlled, approved values
- Document procedures for dealing with actual and potential non-conformances with electronic NCR forms and reportable action items for employees
Using these capabilities, your systems are optimized more tactically, enabling you to identify inefficient areas and reduce wasteful production errors quickly and easily. And by lowering instances of poor-quality outputs, your company’s overall cost of quality will be reduced right along with it.
Increases efficiency
Quality management software offers your company a seamless link between processes, documents, and policies. Having these facets of your company linked in tandem with automated quality workflow capability will automatically increase your company’s efficiency when handling your quality process. And of course, the more efficient your production and quality assurance processes become, the more you will save on time and materials spent to create a superior product or service.
Key Pain Points Alleviated by a QMS Software:
| Pain Point | QMS Software Features |
| Generating Management Support of Quality Initiatives |
|
| Inadequate Quality & Production Planning |
|
| Quality Support for Problem-Solving and Continuous Improvement |
|
| Employee Communication and Engagement |
|
| Procedures and Instructions not Written or Updated |
|
| Breakdown in Customer Relationships |
|
| Training and Development |
|
| Supplier Evaluation and Development |
|
Our ENSUR Quality Management software is a comprehensive, easy to use platform for automating your quality documents and processes. Contact us to today learn more about how our Quality Management Software can help you reach and exceed your quality and compliance goals.







.png?width=627&name=ISO%209001%20Audit%20Checklist%20CTA(1).png)
