If your business is still relying on paper-based processes and manual methods of organizing and routing documents, then you know how tricky it can be to ensure quality across the board. Spreadsheets and other physical documents can get lost, damaged, or irrevocably changed—making it difficult for a business to maintain consistency. In 2020 especially, businesses will not be able to afford these slip-ups due to the fact that their competitors are moving to electronic document management to ensure quality control. Those who stick with manual systems will be left in the dust.
Electronic quality control management is an essential part of a 2020 plan for businesses to maintain consistency both internally and externally. Quality control management can be broken down into four main parts. If you want to discover the four quality control components of an electronic system that will keep you competitive in 2020, read on.
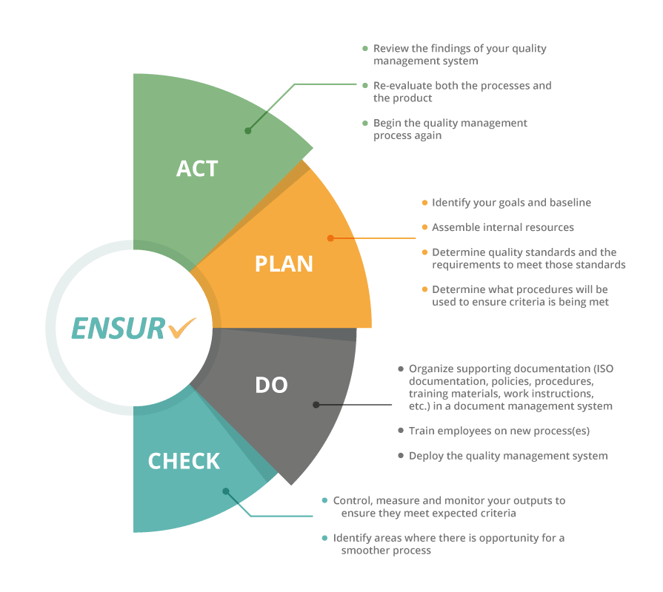
Quality Control Planning
Planning may seem like the obvious first step for a solid quality management system, but it’s one that businesses often skip. You need to take the necessary time to identify and map out your business’s goals for both the short and long-term, so you know what you’re aiming for. Your quality management system will be able to work in tandem with your business’s strategy if goals are clear. Your planning should determine the following:
- What your quality standards are
- What is required to meet those standards
- What procedures you will put in place and follow to ensure you meet those standards
Be sure to start by defining your business’s version of success. From there, you should also consider:
- If you have any legal standards or regulatory requirements your business has to abide by.
- Who is responsible for handling each part of the quality control process from beginning to end.
- How often you will assess your operations to identify areas for improvement.
- If you have any stakeholders that have expectations and priorities you need to account for.
Quality Control
After you’ve completed the strategy and planning phase of quality control management, the actual quality control phase begins. This stage involves making sure what you determined in the planning stage is doable and realistic for your business. At this point, you will make sure that the baseline you created in the planning stage has been met, and you should try to correct any mistakes.
Catching errors early on will save you time and grief the further you get into the quality control process. Examine all sides of your product and gather the data you’ve collected in this inspection process. Display that data so that anyone involved in this part of the process can easily analyze it. Display options include run charts, histograms, and cause and effect displays. You’ll be able to use your document management system to disseminate and share this data with your entire team.
Quality Assurance
This phase involves reviewing the quality management manufacturing of products and/or the delivery process of goods and services. In contrast to the quality control phase where you reviewed the actual goods and services, the quality assurance stage looks at how products are made and delivered. This requires you to evaluate the source of the manufacturing process so you can catch any errors before they risk making their way to customers.
During this phase, you’ll want to constantly refine your process to ensure quality control at every step of manufacturing and delivery. This involves performing audits, which you will need to do regularly. The following steps are a basic outline of how to go about this stage:
- Look at the standards you created in the quality planning stage of your quality control management to confirm that operations match what you mapped out in that stage.
- Ensure your operations are meeting your compliance and regulatory requirements.
- If you discover mistakes, take stock of how they occurred and what you can do in the future to prevent them from happening again.
- If you believe any parts of the process could be better, identify those for future improvement.
Ideally, these regular audits are not conducted by anyone on your internal team but rather an independent, third party.
Quality Improvement
This review stage involves a thorough investigation of all the data you’ve collected throughout the quality control process. Quality improvement means a constant evaluation of what you’ve discovered about your operations. To seal the quality control management process, you’ll use this stage to improve for the future. Here, you’ll collect the data you’ve gathered and look again at the product itself, as well as the processes involved in manufacturing and delivering it (remember always to be evaluating these parts with compliance and regulatory requirements in mind). Then you’ll begin this entire process over again from the beginning of the quality control cycle. The constant improvement required of this phase will ensure your business gets better and better.
Want to Go Electronic for Your Quality Management?
Software-based quality management systems can streamline, organize, speed up, and improve your quality control. DocXellent is the industry expert provider of document management software that enables your business to access and control all your documents in a digital setting.
If you’re looking for the most effective quality control, use a document management software system to integrate with your existing tools. DocXellent’s software is configurable and compatible with whatever your business is already using so you can make your quality control process easy, fast, and efficient.
DocXellent can help you:
- Track the successful completion of any tasks you assign during the document’s lifecycle and assign those tasks at the correct times.
- Create default requirements for any review or approval standards.
- Tailor data entry forms, so you know you’re gathering all the necessary information.
- Let relevant parties know when their action is required to further the process.
- Provide easy access to standards and plans through a searchable database.
- Keep employees and management up to speed on the process through easy document distribution.
- Keep only the right eyes on each document with permissions and access restrictions.
The right document management software is an essential component of quality control management. Today’s businesses are looking at how to integrate document management software into their 2020 plans for quality control. Is your company competing on their level? Contact DocXellent today to make your quality control process the most effective, efficient, and helpful for your business in the coming year.




























