If you want to ensure you are providing consistent products or services throughout your organization, you must implement an ironclad quality management system, or QMS. Quality control management ensures that what your company delivers, and the delivery processes it uses are cohesive and that every business phase of the organization focuses on the same goal.
When broken down, quality control management can be segmented into four key components to be effective: quality planning, quality control, quality assurance, and quality improvement.
In this article, we'll take a closer look at these four crucial steps, as well as provide insight into how a QMS software can make the job easier.
Quality Control Planning
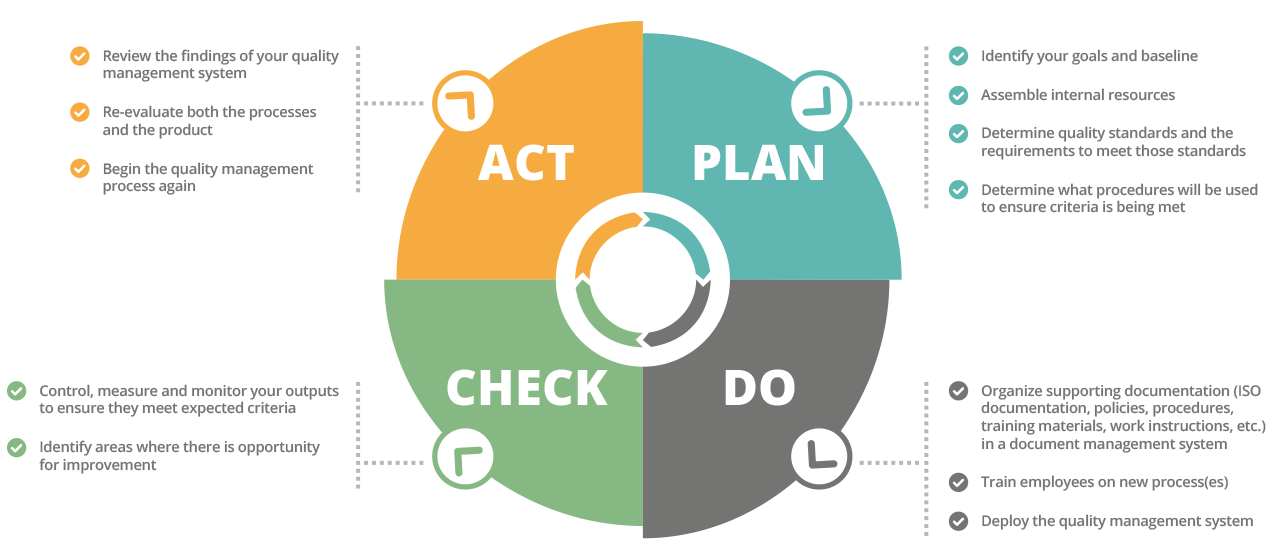
The first step of quality management is planning. You need to take the time to identify your goals and what you want your baseline to be. You should determine what your quality standards are, the requirements necessary to meet these standards, and what procedures will be used to check that these criteria are being met. In this planning stage, you will want to consider:
- What your stakeholder’s expectations and priorities are, if applicable
- What your company’s definition of success is
- What legal standards or requirements are in place that must be abided by
- Who will handle each role in the quality management process (supervision, testing, etc.)
- How often processes will be evaluated for improvement
Quality Control
Once you have a plan in place, quality control comes into play. This is the process of physically inspecting and testing what you laid out in the planning stage to make sure it is obtainable. You need to confirm that all the standards you have put into place are met, and you need to identify any mishaps or errors that need to be corrected. The sooner you can catch these errors, the better. As such, you should be paying attention to all aspects of the product, including both the materials used and the process of putting them together.
Once the inspection data has been collected, it should be displayed in a way that makes it easy to analyze. You can create histograms, run charts, or cause and effect displays, and then easily share them through your document management software to make sure everyone has access to them.
Quality Assurance
While quality control involves inspecting the actual products or services in the field, quality assurance is reviewing the delivery process of services or the quality management manufacturing of goods. By inspecting your goods or services at the source, you can catch mistakes before they reach the customer. You can also fine tune your processes to prevent errors in the future. When reviewing your product or service during this stage of quality control management, you will want to follow these steps:
- Confirm that everything is operating as it was agreed upon during the quality planning stage
- Measure how effective your pre-determined processes are and confirm that all compliance needs are being met
- Take note of any lessons learned
- Identify areas where there is an opportunity for a smoother process
To be effective, quality assurance must be completed regularly through independent audits. For the best results, have the audit completed by a third-party that is not financially or emotionally invested in the outcome.
Quality Improvement
Finally, after completing the quality control process, you need to thoroughly review your findings and come up with a way to improve your methods going forward. Quality control management is fruitless if you are not willing to make changes when they are necessary. The desire for continual improvement is the goal for every successful company. So, gather all your data, re-evaluate both the processes and the product—always keeping compliance in mind—and then begin the quality control management process again. With each cycle, you will end up with a better product, happier customers, and more profit in your pocket.
What are the Main Components of a Quality Management System?
There are 4 main components of every Quality Management System (QMS). They are:
- Quality Control Planning: Identifying your quality goals and standards, the requirements necessary to meet these standards, and what procedures will be used to check that these criteria are being met
- Quality Control: The process of physically inspecting and testing what you laid out in the planning stage to make sure it is obtainable
- Quality Assurance: Reviewing the delivery process of services or the quality management manufacturing of goods
- Quality Improvement: Thoroughly review your findings from the last 3 components and come up with a way to improve your methods going forward
A QMS like ENSUR helps you streamline this process; organizing, accessing, and controlling all of your important documents.
What are Quality Assurance Components?
Quality assurance is the process of reviewing the delivery process of services or the quality management manufacturing of goods. By inspecting your goods or services at the source, you can catch mistakes before they reach the customer. When reviewing your product or service during this stage of quality control management, you will want to follow these steps:
- Confirm that everything is operating as it was agreed upon during the quality planning stage
- Measure how effective your pre-determined processes are and confirm that all compliance needs are being met
- Take note of any lessons learned
- Identify areas where there is an opportunity for a smoother process
How ENSUR QMS Can Help
Total quality management is a complex task that must remain organized to be effective. Our ENSUR Quality Management System can streamline the process of organizing, accessing, and controlling your important quality documents. Whether you are selling a product or providing a service, our ENSUR software can help you with your quality control needs.
Check out our other resources on this topic:
- The Ultimate Guide to Implementing Quality Management
- Best Practices from an Expert on the Quality Audit Process
- 6 Tips to Effectively Manage Your CAPA Workflow
- Why Quality Is the Most Essential Part of the Manufacturing Process






























