-png.png)
For many companies, establishing a Quality Management System (QMS) is imperative as it not only improves overall productivity and quality of products and services, but also ensures compliance. A comprehensive QMS takes a critical look at your internal policies, processes, and procedures to help you increase efficiency throughout production, development, and service. But, managing those processes and procedures can be time-consuming and overwhelming, especially when done manually. Implementing a QMS within your organization doesn’t have to be stressful or complicated. This article will give you 5 tips to help effectively implement a comprehensive quality management system at your company.
- Define your quality objectives
Quality objectives describe your company’s goals for the value level of your products or processes. If you plan on implementing a QMS, you need predetermined base-level targets to compare your actual finished goods to once they are produced. These objectives hold both your company and employees accountable to meeting a measurable value standard. There is a wide range of quality objectives, and they can range from department-specific, to ones that pertain to specific products or processes. Here are some examples of objectives to consider for your company and products:
-
-
- Defects
- Efficiency
- Performance
- Reliability
- Accuracy
- Customer service
- Employee safety
-
- Determine your training needs
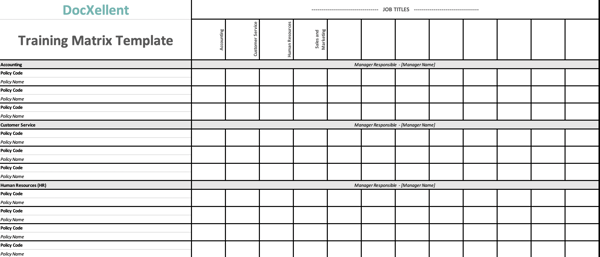
In order to achieve your quality goals, your employees need to be trained on the policies and procedures that make up your quality management system. One of the most overlooked items of any quality plan is the adherence to policies and procedures by the people it’s written to protect. Moreover, one of the most targeted areas of a regulatory audit is the training records of everyone in the company that has a need to know and understand what they do and how they do it.
Developing a training matrix is one of the easiest and most organized things you can do for to assist in your compliance efforts. Establishing employee groups by department, job titles, having detailed job descriptions, identifying supervisors and subordinates are the employee piece. Then marrying that information with SOPs, policies and actually training curriculum per job title is the tedious but essential part. This can easily be done using a spreadsheet.
- Monitor and implement activities to improve your performance
Regularly validating your QMS is a very important part of your quality processes. The act of validating involves reviewing the delivery process of your services or the quality of your finished products to make sure they are meeting your pre-defined quality standards. By inspecting your goods or services at the source, you can catch mistakes before they reach the customer. You can also fine tune your processes to prevent errors in the future. When reviewing your processes in order to validate them, you will want to follow these steps:
-
-
- Confirm that everything is operating as it was agreed in your originally defined quality objectives
- Measure how effective your pre-determined processes are and confirm that all compliance needs are being met
- Take note of any lessons learned
- Identify areas where there is an opportunity for a smoother process
-
- Employ corrective and preventive measures
Corrective Action Preventive Action (CAPA) refers to the process of inspecting and resolving the cause of identified non-conformances within your production processes. Having a well-structured and organized CAPA process is vital for any business, especially one that is implementing a QMS. If your quality process is lacking in any way, CAPA solutions allow you to pinpoint an issue and solve it, preventing it from happening in the future.
- Implement a Quality Management Software
As important as implementing a consistent quality process is for every company, many businesses still find it difficult to maintain all the moving parts and pieces. Manually tracking every aspect of your quality program and ensuring that your employees are trained on relevant quality and safety procedures can be a daunting task. However, moving your quality management processes into an electronic, software-based quality management system can streamline, organize, speed up, and improve this process. These systems allow you to manage:
-
-
- Incidents: create waivers, investigations, complaints and deviations that may arise through audits, product returns, and customer reported issues
- CAPAs: easily create corrective action and preventive action requests, track nonconformities, collaborate on and perform root cause analysis, develop appropriate Action Plans, link CAPAs to any other quality item within the system and attach supporting material such as videos, audio, and email
- Change Controls: get approvals to change current content with changes requests and manage changes to multiple documents with a change order so they are reviewed, approved and made current in a synchronized group, identify reference documents, categorize your changes, document the reason for change, attach affected documents through unique change links, and create default review/approval routings for different categories of changes
- Read & Acknowledge Training: define training requirements, manage rosters, test comprehension and track results
- Review and Approval Routing: Automate review and approval of documents and processes by necessary stakeholders
- Notifications: Set-up automated notifications for workflow actions required by assigned individuals or groups
- Action Items: Assign Action Items to specific employees, departments, or teams and track and report on completion
- Manage non-conformances, deviations, waivers, out of specification and more
-
DocXellent’s ENSUR QMS provides the tools necessary to take your quality program to the next level in order to be more efficient, competitive and compliant. Contact us today to learn how ENSUR can optimize your quality management system.