The 2023 PDA/FDA Joint Regulatory Conference was a highly-anticipated gathering for pharmaceutical professionals, regulatory experts, and industry stakeholders. Tailored specifically for professionals in the pharmaceutical and regulatory sectors,...
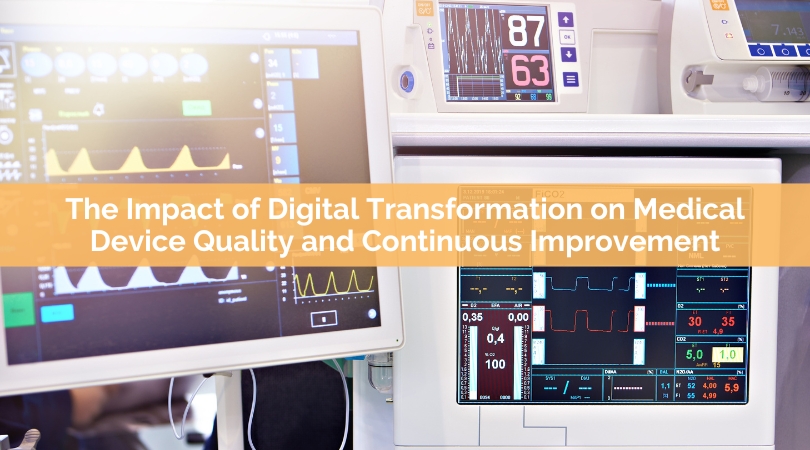
The medical device industry has undergone significant changes in recent years due to the rise of digital transformation. Digital transformation has become a buzzword in the industry, but it is much more than that. Digital transformation is the...
Tags: medical device document control, business continuity, medical device, medical device regulations
For medical device manufacturers, meeting compliance standards is an essential piece of the quality process. One of the most important of these necessary regulations is FDA 21 CFR Part 820, which ensures that medical devices are safe and effective....
Tags: FDA Compliant Document Control Software, medical device regulations, FDA 21 CFR Part 820
Over the next couple of months, the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) is expected to roll out new guidance that will alter the way medical device products are regulated. Preparing for this change may be...
Tags: regulatory, medical device, medical device regulations, UK, UK regulations































-png.png)